|
|
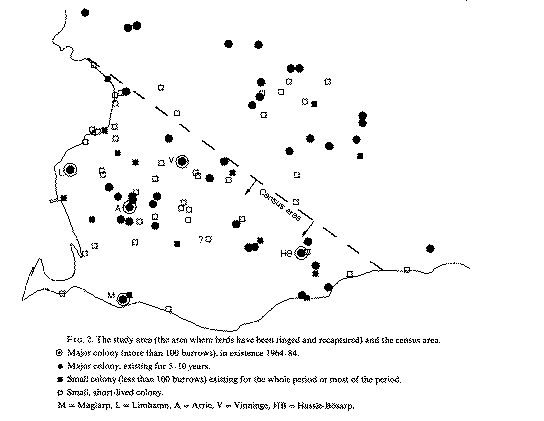
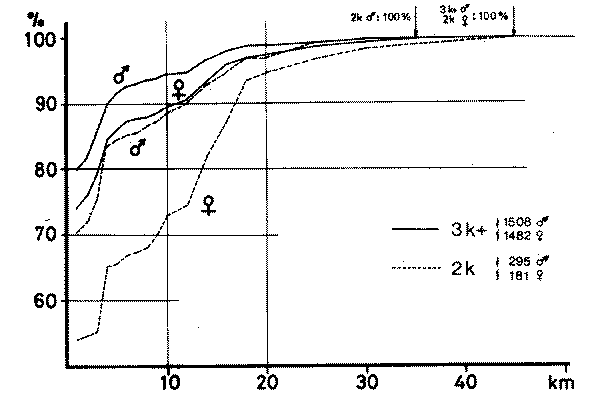
Sex ratio in Sand Martins (Riparia riparia) in relation to hatching date and population level |
| PERIOD OF INCREASE "First brood", ringed 23.6-20.7 | sex ratio | G-test | PERIOD OF DECREASE "First brood", ringed 23.6-20.7 | sex ratio | G-test | |
|---|---|---|---|---|---|---|
| Controlled as: | MALE: 247 FEMALE: 194 | 1.27 | G=6.13; 1 df p<0.02 | MALE: 144 FEMALE: 71 | 2.02 | G=25.28; 1 df p<0.001 |
| PERIOD OF INCREASE "In between" broods, ringed 21.7-30.7 | sex ratio | G-test | PERIOD OF DECREASE "In-between" broods, ringed 21.7-30.7 | sex ratio | G-test | |
|---|---|---|---|---|---|---|
| Controlled as: | MALE: 112 FEMALE: 44 | 2.55 | G=29.73; 1 df p<0.001 | MALE: 51 FEMALE: 43 | 1.19 | n.s. from 50:50 |
| PERIOD OF INCREASE "Second brood", ringed after 30.7 | sex ratio | G-test | PERIOD OF DECREASE "Second brood", ringed after 30.7 | sex ratio | G-test | |
|---|---|---|---|---|---|---|
| Controlled as: | MALE: 80 FEMALE: 56 | 1.43 | n.s. from 50:50 | MALE: 40 FEMALE: 35 | 1.14 | n.s. from 50:50 |