|
|
The shift of SW Scanian Sand Martins (Riparia riparia) from colonies to roosts in late summer |
Sand Martins have "options" in the proper sense of the word only in those parts of the distribution, where two (or more) broods are raised. When compared, single-brooded and multi-brooded Sand Martins appear almost as two different species. The social life of multi-brooded birds reaches a higher level of complexity than that of single-brooded, since only the former are capable of exploiting the possibilities presented by a division of labour between sexes, which is present as a pre-condition during all circumstances. The male makes most of the digging and guards the female, so he will spend much energy in the early phase of the breeding, while the female is saving her energy and nutrients for eggs and brooding. The female alone has a developed brood-patch, a fact that predestines her for brooding overnight (and as a matter of fact much of the day as well). She is relieved by the male in the morning, when temperatures rise and insects begin to emerge. This is the daily routine in single-brooded and multi-brooded birds alike.
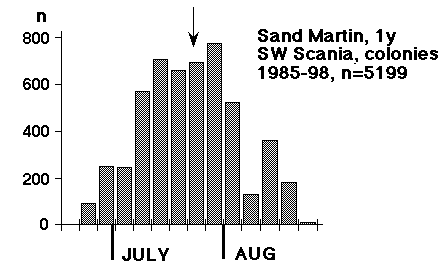
Before going on, another matter connected with breeding must be touched upon. In various contexts, e.g. in Emlen & Demong (1975), it has been suggested, that pronounced synchronization of breeding within colonies or sections of colonies should be connected with some kind of advantage: "An individual that fledges either early or at the peak of synchrony will emerge to find a steady stream of other birds traveling to local ephemeral concentrations of food". It's an undeniable fact, that a group of birds, which jointly turn to a cliff and excavate burrows there, will be highly synchronized when it comes to egg-laying, hatching and fledging of young. But in the same "colony" (quarry, gravel-pit) there will in most cases be subcolonies that are delayed relative to each other (digging, brooding and feeding birds within a distance of 100 m), and when a larger area is covered, I have often found that close-lying colonies (2 or 3 km apart) are seldom synchronized. They draw breeding birds from the same pool, and the delay between them may amount to as much as 10 days. These differences are even "desirable"; due to the capricious summer climate of NW Europe (particularly Scandinavia and the British Isles) spreading of risks is imperative for Sand Martins. A populations should not "have all its eggs in one basket". This spreading of risks, which is fairly evident from Fig. 1 - no other species, maybe with the exception of Panurus biarmicus, ejects offspring in this way over such an extended period - is attained on one hand through a protracted spring migration, where many birds do not turn up at the colony until early June, on the other through natural "upsets", caused by predation and sandslides. From the first excavations in May till late July - for 2 to 2 1/2 months - the birds counter each reverse of this kind by forming a new group and entering breeding anew. There is no overall synchrony, just a great turmoil, and there will always be birds at hand e.g. to point out "ephemeral concentrations of food" to young birds.
Where there is a sequence of broods, the fledged birds from the first brood will leave the colony "at some point of time", accompanied by the males that fathered them; this departure continuously drains the stock of males in the colony. At the same time the female will mate a new male, as long as extra males are available. Next, the whole breeding procedure will take a new turn. The present paper deals with multi-brooded Sand Martins and an aspect of their phenology: the time schedule of the transition from breeding to migration. As is evident from the above, there are two problems that must be solved one way or another within the framework of the breeding, the first one concerns the no-longer-breeding fraction of the colony and has two main solutions:
The purpose of this beginning was touched upon in the "Introduction": from the start I want to play down the the weight of adaptive categories, adaptive thought, which always comes in afterwards, wise when the event has taken place. My restriction applies to situations that are solved within the framework of the social structure; physiological or physiologically instructed quantities like brood size, fat deposits etc. lie outside the scope of my reasoning). Social choice involving interaction between individuals is not "instructed", cannot be reached by instruction: a choice between alternatives is always solved ad hoc, in situ, and several different alternatives are workable, possible to integrate within the framework of a local population. "The adaptive significance" of synchronization, non-synchronization, early migration, late migration is, when all aspects are taken into account (and: within limits) - nil, zero. Decisive is how the population operates with all its parameters, while advantages obtained by a selfish individual are dispersed like husks to the winds. Now to material that illustrates the transfer of Sand Martins from colonies to roosts and the subsequent migration.
Early summer movements or even early summer migration in a handful of species (adult Reed Warblers Acrocephalus scirpaceus, Sedge Warblers Acrocephalus schoenobaenus, Marsh Warblers Acrocephalus palustris, Icterine Warblers Hippolais icterina, Wood Warblers Phylloscopus sibilatrix and - young Sand Martins) have not been fully elucidated; for reasons of bird protection roosts are little worked before c20 July. Still it is obvious, that the species mentioned "push" a little at the south coast of Scania as early as 15 July, and at least Icterine and Wood Warblers (maybe adults having lost the brood) probably start as early as 10 July. Once this state of things is accepted it is easier to be ready for the fact that Sand Martins also begin to migrate somewhere in July, and that we probably lack material (catches from roosts, overseas recoveries) from this initial period, at least as far as Sweden is concerned. Fig. 3 shows all retraps of juvenile Sand Martins between colonies and roosts and all local retraps of juveniles ringed at roosts. A dozen colonies are involved, from the Isle of Hven in Öresund to Maglarp near Trelleborg (but the major part of the material comes from the colony Maglarp and the roost Klagshamn). On average 8 ± 5 days after having been ringed in a colony juveniles were recaught at roosts, two birds even in the evening of the same day. The first juveniles to be caught at roosts (23 July) had been ringed in colonies on 17 July. The median catching date for juveniles at colonies is 23 July, so there is no return from roosts of the opening 40 % of the total catch. In all probability early juveniles are capable of leaving the colony and spend the night in roosts from the first decade of July, and the first males probably migrate by the same time as well. Equally interesting is the fact, that juveniles obviously do not linger at roosts: six birds were retrapped on average 4.5 days after being ringed at a roost, and no juvenile - neither from colony nor roost - was retrapped twice at a roost. Fig. 3 illustrates hurry, quick throughput.
In addition there is a 1y bird, ringed at Flommen, Falsterbo (55.23 N, 12.50 E) 22.7.88 and retrapped at Maglarp in 1989, and a 1y from Klagshamn 16.9.93, retrapped at Maglarp in 1995. So juveniles of local origin visit SW Scanian roosts at least between 22 July and 16 September. But not all juveniles are equally eager to leave the colony (with the burrow protecting against rain in the night); a not negligible amount may choose to stay there for a fortnight or more. A selection (part of the data available have still not been analyzed) of such retraps are shown in fig. 4. 31 juveniles stayed in the colony on average another 13 ± 5 days after being ringed. In these cases there seems to be a slower pace than in the roosts, one reason may be that this material contains younger, less developed birds, or that there is a bias in favour of rainy summers. The possibility that the disturbance connected with ringing speeds up the departure for roosts, must be considered as well. (So far this is unclear). A third alternative is for juveniles to join another colony and spend the nights in one of its unoccupied burrows. All retraps belonging to this category are listed in Table I, the average stay was 13 ± 8 days, i.e. of the same length as the longest stays in the birth colony. Finally, a double control demonstrates that not all adult males have committed themselves when they show up at roosts:
In 1995 a proportion of the female population at Maglarp (less than 10 %) must have raised three broods, and males were in short supply towards the end of the season. This particular male may have accompanied young belonging to the second brood to Foteviken and then returned back in order to assist in the raising of a third brood.
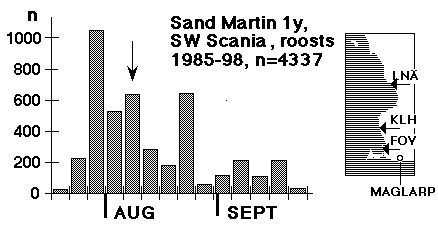
The aim of the gathering at roosts seems to be to attain some minimum flock size (say: at least one thousand individuals), hence the strange pulsating character of overall numbers that can be discerned in fig. 1 as well. This diagram suggests a culmination of a "first brood" (or first + replacement) shortly before 1 August and a consecutive culmination of a "second brood" by 20 August, in addition the distribution has a very typical feature of a long-distance migrant: it is skewed to the left (Dorka 1966). In the case of Sand Martins, the distribution of autumn migrants will never attain the smooth, continuous character which is seen in species like Willow Warbler and Yellow Wagtail. On a calm and warm evening in August, 3000 Sand Martins may roost at Foteviken or Klagshamn, the following evening, with unchanged weather, a mere 200. A contingent of birds has left the area, a new contingent is building up. (But sometimes obtrusive Sparrow Hawks may lie behind a change of roosts). In the end the time needed to build up a minimum flock size is the crucial point for the individual, deciding its length of stay in the roost. In all likelihood this stay will be shortest around 1 August, when the throughput of birds reaches a maximum. 1. The pace 2. The options 3. Juveniles should "follow" 4. A model for the dynamic interaction of Sand Martins prior to the autumn departure 5. Objections from adaptive thinking A preliminary summary: Facts and interpretation put forward here may be summed up as follows: Roosts (primary roosts) lie within the daily radius of action of colonies. The first male to spend the night in a roost together with juveniles from the first brood will trigger the landslide of migration. The small pioneering group will attract other birds on the verge of taking the same step, at the same time the reduction of the number of males in the colony will be perceived as a change in the overall signal level: suddenly a female flying out for a breath of air will no longer be pursued by four males, but only by three or two. At roosts the intense display in the evenings combines to prepare the birds involved for migration: a large flock seldom goes on with this kind of exercise for more than a week, after that most of the birds are gone - but at the same time the next flock is already forming. This reasoning is supported by Fig. 3; birds seldom linger at the roost, it serves as a whipper-in for migration. The adult bird that has been touched by the "spirit" of roosts is lost to breeding - this most certainly holds good for all females, and probably for most males as well. At the same time one must not shut one's eye to a movement in the opposite direction; if the breeding "task" did not have its attraction, there would be no September broods of Sand Martins in South Sweden.
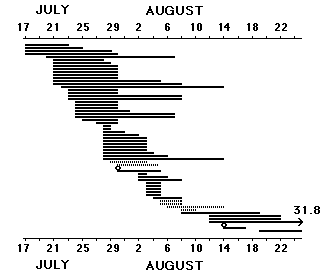
Fig. 3 Time elapsed between ringing in a colony and retrap in a roost for 57 juvenile Sand Martins (black bars) and between ringing and retrap in a roost for 6 juvenile Sand Martins (striped bars). 0 = caught in colony in the morning and in roost in the same evening.

Fig. 4 Time elapsed between two retraps of juvenile Sand Martins staying for some time in the birth colony. Only a selection of the existing material (the longest stays). n=31
Ringing date Distance (km), direction Interval (days) 25.6 18 NW 26 25.6 28 W 9 27.6 30 ENE 14 1.7 4 SE 13 3.7 13 SSW 5 3.7 48 SSW 16 4.7 23 NE 10 7.7 4 NW 26 11.7 12 SSW 1 14.7 7 ESE 4 18.7 0.5 SW 1 hour 20.7 12 SSW 11 20.7 3.5 W 15
In addition there are two controls of juveniles born at some distance, both caught at dawn in colonies,
and two juveniles of more distant origin which were controlled at a remarkably early date in SW Scanian roosts,
and finally a juvenile returning to a colony and spending the night there, after having been caught and ringed at a roost:
Occurrence of adult birds at roosts; adult departure
Adults are in minority at autumn roosts; their share of the total catch being 10 - 20 %. Between 1985 and 1998 403 adult Sand Martins were ringed at the three sites shown in fig. 2. From early August the brood patch is beginning to retrograde in females, and many can no longer be separated from males, so, from mid-August only females with distinct brood patch are sexed. On the other hand sexing is no problem in the case of retraps (n = 51), where birds were caught and examined in the breeding colony. We do not catch at colonies until all females have developed a brood patch, from c10 - 15 June. The distribution of ringed and retrapped adults at roosts is summarized in Table II:
Sex; n Interval Median males (ringed): 134 20.7-29.8 30 July females (ringed): 152 20.7-12.9 5 August unsexed (ringed): 117 25.7-17.9 19 August males (retr.): 28 20.7-19.8 31 July females (retr.): 23 23.7-25.8 6 August
Discussion
A popular and frequently utilized picture in fiction and poetry concerns the "gathering" of hirundines in autumn and their final departure for the South in close order. This picture is in poor agreement with the true state of affairs among Sand Martins; as a matter of fact the "removal" of SW Scanian juveniles and adult males is launched very early in the season - 15 July may serve as preliminary starting date, but the first birds probably leave as early as 5 July - and after the first departures the process continues at a high and steady pace. The difference between the medians of Fig. 1 and Fig. 2 gives an approximate value of the age of migrating juveniles: they have been fledged for no more than c17 days. The same pace has been recorded further south; according to Mead & Harrison (1979b) the fastest British juvenile flew from Fife, Scotland (ringing date 24 July) to Bordeaux, France in four days - a distance of 1242 km! (A similar haste seems to occur among juvenile Marsh Warblers; judging from the moult state of body feathers many of these birds have been fledged for little more than 14 days when they appear - independent of adults - on the S coast of Scania. ref.: B. Petersson). The first adult male Sand Martins probably leave the country by 15 July, while colony life claims most females till well into August. By September 1st practically all breeding adults (except for a minor group in some years struggling with a third or late replacement brood) have left Scania, and most birds, adults as well as juveniles, visiting Scanian roosts in September seem to be of northern origin. (Two coming papers will bring up these matters; one dealing with the presence of sexes and age-classes in the colony, one with the origin of juveniles ringed in September).
The breeding of multi-brooded Sand Martins appears to take place in a chamber with rigidly instituted walls: the whole matter must be finished within the scope of three summer months, and as an extra embarassment a Swedish (NW European) June is frequently too cold for hirundines, the birds get along without impetus, the young do not exert their growth in the burrows. When summer finally makes its appearance and the raising of young flows, breeding picks up speed. The particular breeding system of multi-brooded Sand Martins (Cowley 1983) is directed upon achieving a maximum output of offspring during this period. In addition to that there is a second precondition, however: a kind of ruggedness in juveniles, an ability to look after themselves. Sand Martins profit from this ability when juveniles are passed on to an intermediate station at the roosts, the birth colony or some other colony, where they can benefit from information carried by the adults. Here, after a surprisingly short time they have to get on without individual care from the adults. In all cases, in males, females and juveniles, the key to success is following, an associative process, where males take up a central position:

Fig. 5. Options of different groups after the first brood is fledged. Sovvass = roost, andrakull = second brood, flyttning = migration.
The male has know-how: he knows when and where females are accessible (by force of the pursuit flight), he can lead small young from the birth colony to a neighbouring colony or to a protected site and continue to feed them there, and he can guide well-developed juveniles to the roosts. By virtue of this capability he has a key role as informer. At the same time there is still a choice for juveniles, they may obtain know-how in alternative ways. If the young bird isn't ready to leave the protective environment of the colony, it may try to cope on its own within a colony. In both cases the solution to the problem is to "follow the model", the basic pattern is associative. Purpose in the shape of e.g. shelter from predation never touches this very fundamental process (and it must be fundamental because of the total inexperience of juveniles, it is the direct successor of embryonal development); formation is un-intentional and always possible to repair or reconstruct at this basic level. The final result of the dynamic interaction on the part of juveniles is their final departure for wintering grounds (for dynamic model: see below).
For the modelling I will resort to the elementary catastrophe theory that can be found in the theory folder from August 2001. The theory must be read (and understood) here before the following can be grasped. Necessary previous knowledge is some idea about function theory from the high school level, in addition maybe some "sense" for 3D (and 4D) geometry; if these requisites are not met with the following might as well be left out by the reader. Set theory, with some knowledge of linear transformations, quadratic and cubic forms, will be necessary for further studies.
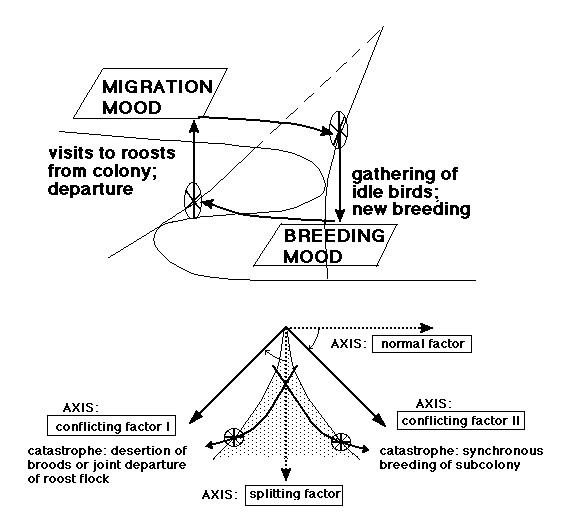
Fig. 6. Model for the dynamic interaction between Sand Martins, leading to prolonged breeding (second and third broods) or early departure. In the upper part of the figure the hysteresis pattern is indicated. The cusp is shown shaded; two paths, one leading to departure, one to new breeding are shown below. The suggested turn of splitting/normal factors (dashed) into conflicting control factor axes situated on both sides of the cusp is indicated.
The proposal that two or three (or maybe even ten) basic patterns are "workable" within the context of a Sand Martin population is confronted with the objection: Why then do the early juveniles migrate so early, and why do some breeders migrate so early, instead of putting their money (their ability, their fitness) on more offspring? Early fledged juveniles have better survival than late fledged (Persson 1987), and it is tempting to suggest that their departure creates living space for replacement and second broods by not pinching from the food supply of the colony. In Scania this objection is unvalid; all roosts are situated within the daily radius of action (10 - 15 km) of foraging adults from several major colonies.
LITERATURE
This article was written by Christer Persson, first English version on the web: 10.4.99, corrected 2.5.99, addition 27.6.01. There will be changes and additions to the text in a process of reviewing. Roosts were worked by Kerstin Norrman, Per Nothagen and Peter Olsson (Foteviken, Klagshamn) and Ulf Lundwall (Löddesnäs), colonies mainly by Christer Persson.